-40%
Multi-parameter 12'' Vital Sign Patient Monitor ECG NIBP RESP TEMP SPO2 PR FDA
$ 256.08
- Description
- Size Guide
Description
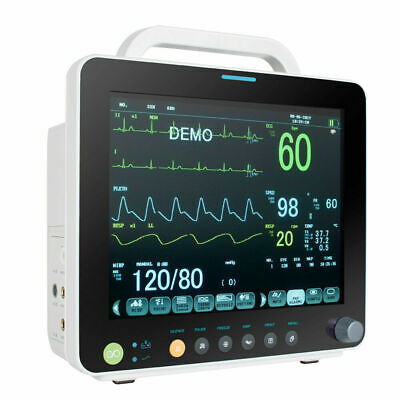
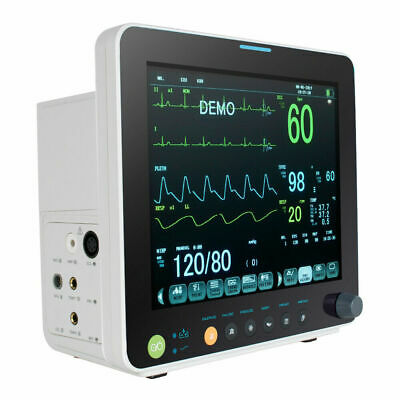
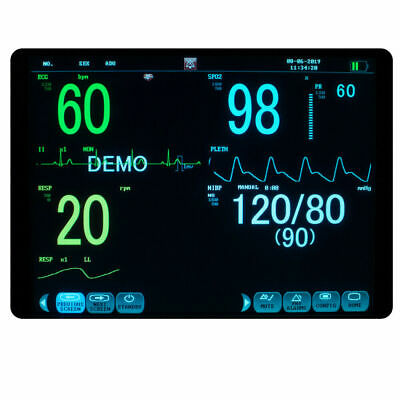
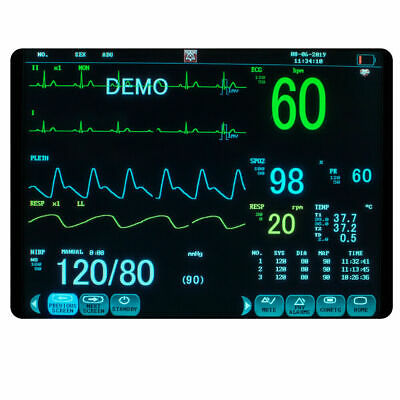
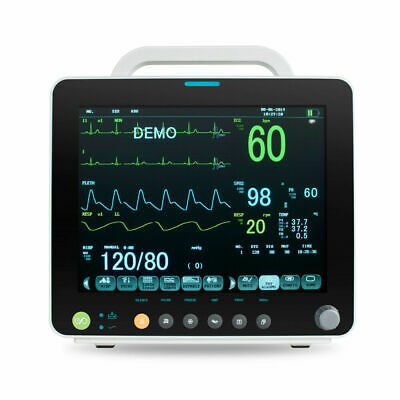
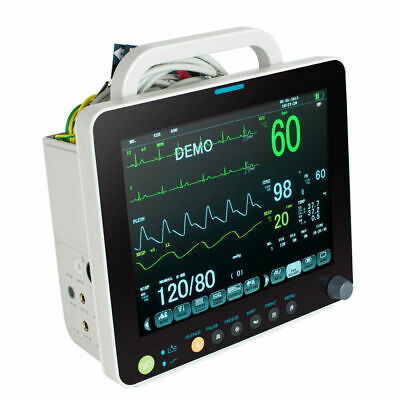
Description:Portable Multi-parameter
Patient Monitor is adaptable to adult, pediatric and neonatal usage. It can monitor vital signals as ECG, Respiratory Rate, SpO2, NIBP, TEMP and IBP.It integrates parameter measuring modules, display and recorder in one device, featuring in compactness, lightweight and portability. Replaceable built-in battery facilitates transportation of patient.
Features:
12"
high resolution color TFT LCD display.
6 Standard parameters:ECG, RESP, NIBP, SPO2, TEMP, PR.
Can be arbitrarily set the alarm limit, automatic sound and light alarm.
The back of the machine is designed with a storage box, which can put some accessories,convenient
for doctors use.
Drug solubility calculation function, Arrhythmia analysis function
.
Built-in lithium battery,AC and DC,can provide long-term monitoring after power failure
.
Widely used in the Department of internal medicine, Dental practices (both pediatric and adult), oral surgery centers surgery, operation room,obstetrics and Gynecology, pediatrics, emergency center,ICU,CCU
.
Specification:
ECG
Lead Mode
:
5 Leads (
R, L, F, N, C or RA, LA, LL, RL,V)
Lead selection
:
I, II, III, avR, avL, avF, V,
Waveform
:
2 ch
Lead mode
:
3 Leads ( R, L, F or RA, LA, LL)
Lead selection
:
I, II, III,
Waveform
:
1 ch
Gain
:
´
2.5mm/mV,
´
5.0mm/mV,
´
10mm/mV,
´
20mm/mV, auto
HR and Alarm
Range:
Adu
lt:
15 ~ 300 bpm
Neo/Ped
:
15 ~ 350 bpm
Accuracy
:
± 1% or ± 1bpm,which great
Resolution
:
1 bpm
Sensitivity
:
> 200 (uV P-P)
Differential Input Impedance
:
> 5 MΩ
RESPARATION
:
Method
:
Impedance between R-F(RA-LL)
Differential Input Impedance
:
>2.5 MΩ
Measuring Impedance Range:0.3~5.0Ω
Base line Impedance Range:0 – 2.5 KΩ
Bandwidth
:
0.3 ~ 2.5 Hz
Resp. Rate
Measuring and Alarm Range
Adult
:
0 ~ 120 rpm
Neo/Ped
:
0 ~ 150 rpm
Resolution
:
1 rpm
Accuracy
:
±
2 rpm
Apean Alarm
:
10 ~ 40 S
NIBP
Method
:
Oscillometric
Mode
:
Manual, Auto, STAT
Measuring Interval in AUTO Mode
1, 2, 3, 4, 5, 10, 15, 30, 60, 90, 120, 180, 240,480 (Min)
Measuring Period in STAT Mode
:
5 Min
Pulse Rate Range
:
40 ~ 240 bpm
Alarm Type
:
SYS, DIA, MEAN
Measuring and alarm range
:
Adult Mode
SYS
:
40 ~ 270 mmHg
DIA
:
10 ~ 215 mmHg
MEAN
:
20 ~ 235 mmHg
Pediatric Mode
SYS
:
40 ~ 200 mmHg
DIA
:
10 ~ 150 mmHg
MEAN
:
20 ~ 165 mmHg
Neonatal Mode
SYS
:
40 ~ 135 mmHg
DIA
:
10 ~ 100 mmHg
MEAN
:
20 ~ 110 mmHg
Overpressure Protection
Adult Mode
:
297
±
3 mmHg
Pediatric Mode
:
240
±
3 mmHg
Neonatal Mode
:
147
±
3 mmHg
SpO2
Measuring Range
:
0 ~ 100 %
Alarm Range
:
0 ~ 100 %
Resolution
:
1 %
Accuracy
:
70% ~ 100%
±
2 %
0% ~ 69% unspecified
Actualization interval
:
about 1 Sec.
Alarm Delay
:
10 Sec.
Pulse Rate
Measuring and Alarm Range
:
0~254bpm
Resolution
:
1bpm
Accuracy
:
±
2bpm
I.5TEMP measurement
:
Channel
:
1
Measuring and Alarm Range
:
0 ~ 50
°
C
Resolution
:
0.1
°
C
Accuracy
:
±
0.1
°
C
Actualization interval
:
about 1 Sec.
Average Time Constant
:
< 10 Sec.
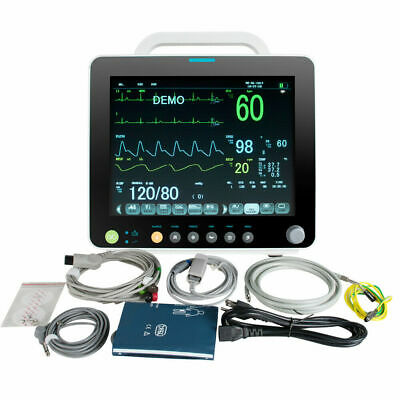
Package List:
1×Patient Monitor
1×
EKG Lead wire
1
×
NIBP Cuff
1
×
NIBP Extension Cable
1
×
SpO2 Sensor
1
×
Temp Sensor
1
×Ground Cable
5
×
Electrode
2
×Fuse
1
×User Manual(CD)
FDA declaration:
""""The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.""""
(Seller name: Rosy Yu City: Beijing State: Beijing Country: China Phone : +86 18663547811)
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892